Go toward the contribution for Asian public health
Company principle
Public Health is the basic condition of Human Security, which is to be given to people equally regardless of race, nationality, and social status. BioShoot sets out for its goal, where it contributes to supporting and improving the health of people through the development and provision of biologicals that prevent or treat infectious and other diseases in Asian countries.

Our mission
Biologicals are the pivot of public health in each country that protects people from the threat of infectious diseases, and the prevention system in the whole of Asian will be robust and solid once Asian neighboring countries improve its own supply system of biologics respectively.
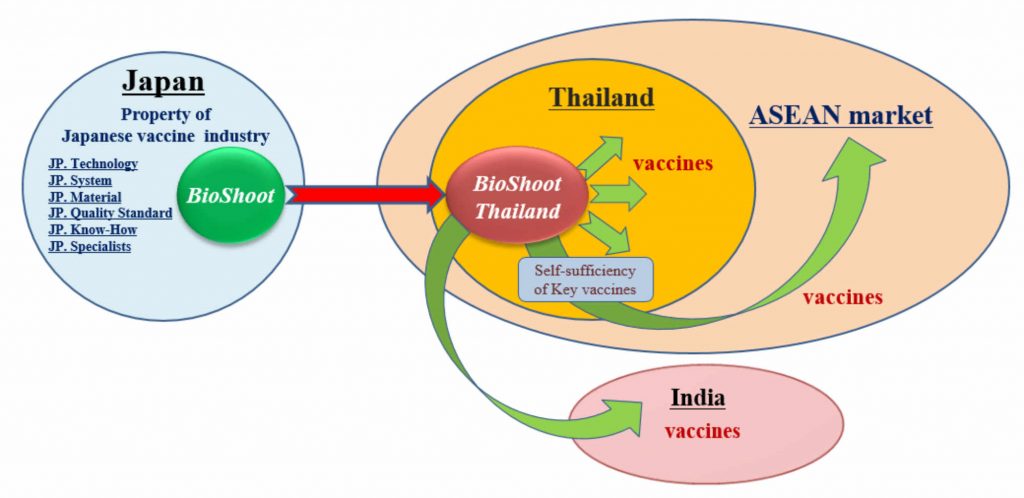
However, the Asian market of biologicals, such as vaccines, are conquered by Western mega- pharma companies and supply volume and price of products do not necessarily match the local needs and the stable supply is not achieved generally. Our mission is to launch the local production system of biologicals in Asia by utilizing the knowledge, technology, and skill for development and manufacture of biologicals that have been developed in Japan, and so that to contribute to improve the public health standard in whole Asia.

About Us
BioShoot was born in 2018 to contribute to the public health in Asian countries by provisioning the safe and reliable vaccines that are manufactured by the production technology of biologics which has been developed in Japan, with the spirit that all of the activities are for the patient benefit.
To achieve our mission, we shall run toward our goal that is to develop and manufacture the vaccines locally in Asia by ourselves, and that is not mere technology support or consulting activities. Then we believe the local independent and sustainable industry of public health will be established in each Asian country.
Company Name
BioShoot (Thailand) Co., LTD.
Date of the establishment
October 19, 2018
Capital
30,000,000 Bht (≒ 0.9 million US$)
Executive
Koichi Yokoi (CEO)
Executive
Hiroyuki Yokote (CSO)
Business contents
Development, production and supply of biological products


Head Office
191 Silom Complex Building, 20th Floor, Room D, D1, D2, Silom Road, Kwaeng Silom, Khet Bangrak, Bangkok Thailand 10500
Tel
+66 (0)2-119-4661
Fax
+66 (0)2-119-4660
Mission
Contribution to strengthening the prevention system of infection in entire Asian region (Global health Security)
Based on the knowledge, technology, and experience of development and production of biological products, that were developed and accumulated in Japan, we will manufacture the Japanese quality of safe vaccines locally in South Asian countries with a large amount and reasonable price, and supply for the Asian market.
Background
It is expected on international cooperation, that the same benefit of health that Japanese people have been enjoying with the vaccines, that are the Japanese research assets, should be given to other Asian people.
Stable supply of vaccine is an important national issue:
- Vaccine is the pivot of protection from the infectious diseases
- Prevention of an epidemic, National security
Stable supply of vaccine is not achieved as a whole in Asian countries
The market mostly rely on the importation
- Supply volume and price do not meet the local conditions.
Concept
Stable supply of vaccines is an important issue in each country from the viewpoint of both national epidemic prevention and national security, but stable supply has not yet achieved as a whole in Asian countries. If a Japanese biologic company developed, produced and supplied locally the Japanese vaccines that meet Asian local needs in terms of quantity, quality and price, it will contribute to implementing the Japanese government’s measure ” Enhancing the infection control and health system in the developing countries” that is one of the measures of the Japanese government policy of “Sustainable Development Goals (SDGs)”, and at the same time, it will also meet the spirit of international cooperation of “Universal Health Coverage (UHC)”
Until now, technical supports of vaccine production to Asian countries have been implemented by private sectors and some results were achieved, however those activities have a limitation of human resources and continuity, and consequently, it has unfortunately not necessarily lead to the successful establishment of local independent and sustainable public health industry.
Start-up plan for local production of the Japanese vaccines in Southeast Asia

Since the subjects of vaccines are mainly children so that requirements of safety level are especially high. Japanese vaccines’ quality and safety have improved quite high through the past social events of side effects that occurred in 1960-1980 in Japan. BioShoot is the specialist group of which members are experts having more than 30 years experiences of R&D and production of vaccines, and the vaccines that BioShoot tends to develop in Asian countries are the products that they had actually developed and been manufactured in the past by themselves so that the vaccines will surely be developed without failures.
To manufacture vaccines locally in Asian countries, and strengthen Global Health Security in the entire Asian region
Main Know-How belonging to BioShoot
1.Mass production of cell culture techniques:
The expression level of the vaccine culturing is lower than other pharmaceuticals, so a large amount of cell culture (10E12-14 cells) technology is necessary.
・Medium composition
・Culture conditions (Temperature, Stirring number, Added nutrient, etc.)
2.Virus mass culture technology:
・Timing of seeding, harvesting, etc
・Purification techniques for high molecular (Suppression of impurities forming, Removal condition of impurities)
・Quality control technology (Main QC tests are Bioassay, which requires high expertise.3Safety net:
・These know-how is limited to special experts, and it takes more than 5 years to learn.
Business
BioShoot will make every effort to protect as many people as possible from the menace of unfortunate infectious disease having the base of Japanese comprehensive technology for biologics, and to pursue the business attitude to be a manifestation of spirit of public health.◎Support and contribution for development , production and supply
◎The fileds we work on
(1)To development, establish production system of biological products
(2) Contract manufacturing of biological products
(3) Integration of technology overlapping regenerative medicine and relating project
(4) The testing business of infectious disease.

CEO
KOICHI YOKOI
Greeting
BioShoot was born in 2018 with resolution for the public that is to contribute to the public health in Asian countries giving the security and safety through the fine quality and production technology of biologics which have been developed in Japan, cherishing its mission that all of the activities are just for the patient benefit.
To fulfill our mission, we shall run toward our goal that we establish development, production and supply system locally in Asia by ourselves, that is not mere technology support or consulting activity. Then we believe the independent and sustainable public health system will be established locally in each country.
We have been in charge of pharmaceutical business in the field of biological products for more than 35 years, and now we have determined to serve for the public health in Asian countries concentrating together with the people who succeed the biological technology which have been developed in Japan during the last 70 years after the world war II and the local associates in each country. CEO Koichi Yokoi
CEO
KOICHI YOKOI
Personal History:
(Inside of the former company)
- 1981: Join “KAKETSUKEN” (The Chemo-Sero-therapeautic Research Institute)
- 1991: Manager of personnel section,
- 2000: Deputy General Manager of Maketing Dept.
- 2004: Deputy General Manager of Research Administration Dept.,
- 2006: General Manager of Research Administration Dept.
- 2009: Board of Directors (In charge of R&D unit)
- 2011: Executive Managing Director, Board of Directors
- 2014: Vice CEO, Vice Chairman
(Outside)
- 2009: Board of trusteeds of Kumamoto Health Science University 2011: Board of Director of The Japanese Vaccine Industry Association
(Some of the business achievement in the former company)
- Registration of Influenza vaccine, DPT vaccine, JE vaccine, Recombinant HB vaccine in south east countries and Korea
- To “S. S. company” , Recombinant HB vaccine export negotiation and conclusion
- From “M company”, MMR-II introduction negotiation and conclusion
- To “S company”, culture & purification methods of Pertussis vaccine licensing out negotiation and conclusion
- To “G company”, Hep A+Hep B combination vaccine patent license out negotiation and conclusion
- To WHO, in charge of expert conference concerning WHO Guideline for efficacy of Pertussis vaccine
- To “V company”, Smallpox vaccine license out negotiation and conclusion.
- With “B company”, Recombinant Coagulation Drug patent exchange and co-development negotiation and conclusion
- To “K company”, JE vaccine export negotiation and conclusion
- From “G company”, Cell culture Pandemic Influenza vaccine license in negotiation and conclusion
- To “V company”, JE vaccine supply agreement concluded
- From “The B. Institute”, Hib vaccine seed and technology transfer negotiation and conclusion
- From “S. I. company”, Pneumococcal vac. 13 valent, License-in negotiation and conclusion
- From “N company” , Rabies vaccine introductio negotiation and conclusion
- With “G company”, cell culture mixed seasonal Influenza vaccine co-development negotiation and conclusion
- To “G company”, Hep A+Hep B combination vaccine patent license out negotiation and conclusion
- To WHO, in charge of expert conference concerning WHO Guideline for efficacy of Pertussis vaccine
- To “V company”, Smallpox vaccine license out negotiation and conclusion.
- With “B company”, Recombinant Coagulation Drug patent exchange and co-development negotiation and conclusion
- To “K company”, JE vaccine export negotiation and conclusion
- From “G company”, Cell culture Pandemic Influenza vaccine license in negotiation and conclusion
- To “V company”, JE vaccine supply agreement concluded
- From “The B. Institute”, Hib vaccine seed and technology transfer negotiation and conclusion.
- From “S. I. company”, Pneumococcal vac. 13 valent, License-in negotiation and conclusion
- From “N company” , Rabies vaccine introductio negotiation and conclusion
- With “G company”, cell culture mixed seasonal Influenza vaccine co-development negotiation and conclusion

Chief Science Officer
HIROYUKI YOKOTE
Personal History:
(Inside of the former company, Kaketsuken)
- 1981: Join “KAKETSUKEN” (The Chemo-Sero-therapeautic Research Institute)
- 1991: Manager of Clinical development Dept,
- 2003: Deputy General Manager of Vaccine Manufacturing Dept.
- 2010: General Manager of Clinical development Dept,
- 2010: General Manager of Clinical development Dept,
- 2012: General Manager of International strategic Dept,
- 2014: General Manager of Development Dept,
- 2014: General Manager of Executive Secretary Office
(Other Experience and Professional Memberships)
- 1993-2005: International Conference on Harmonization (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use
- 2004: Smallpox Vaccine Research Group
- 2007: ASM Biodefense and Emerging Diseases Research Meeting
- 2007: WHO Advisory Committee on Variola Virus Research The 38th World Congress on Military Medicine
- 2010: U.S.-Japan Medical Biodefense Symposium
- 2012: Biologics Forum (Honors) President Award for the Development of “RECOMBINANT ADSORBED HEPATITIS B VACCINE (PREPARED FROM YEAST): Bimmgen” Distinguished Service Award from the Japan Health Sciences FoundationPresident Award for the Development of “FREEZE-DRIED SMALLPOX VACCINE PREPARED IN CELL CULTURE: LC16m8”
(Some of the business achievement in the former company)
- Registration and Development of “RECOMBINANT ADSORBED HEPATITIS B VACCINE (PREPARED FROM YEAST): Bimmgen” in Japan.
- Registration and Development of “Freeze-dried, cell culture-derived Japanese encephalitis vaccine (inactivated): ENCEVAC” in Japan and South Korea.
- Development of “Attenuated Tissue-Cultured Smallpox Vaccine LC16m8” in US.
- Development of Vaccines, Blood products and Monoclonal products in Japan.
- To “HHS/ASPR/BARDA”, Smallpox vaccine Development contract negotiation and conclusion.(Selected Peer-reviewed Publications) Tomoya Saito, Hiroyuki Yokote, et al. Clinical and Immunological Response to Attenuated Tissue-Cultured Smallpox Vaccine LC16m8. JAMA. Vol. 301, No.10 1025-1033, 2009Hiroyuki Y, Yasuhiko S, et al. Safety of Attenuated Smallpox Vaccine LC16m8 in Immunodeficient Mice. Clin Vaccine Immunol. 21(9):1261-6, 2014 Other (12)
(Oral Presentations and Poster Presentations)
- H. Yokote, Y. Shinmura, et al. LC16m8, an attenuated smallpox vaccine protects mice against lethal orthopoxvirus challenge from the early-stage to the long-term after vaccination. IUMS 2008 Istanbul Convention Congress of Virology. Aug. 10-15, 2008 H. Yokote, Y. Shinmura,et al. . Efficacy and Safety Evaluation of Attenuated Smallpox Vaccine LC16m8. International Meeting on Emerging Disease and Surveillance, Feb. 23-25, 2007 Other (26)

Director of QA Department
YOICHI OGATA
Personal History:
(Inside of the former company, Kaketsuken)
- 1978: Join “KAKETSUKEN” (The Chemo-Sero-therapeutic Research Institute)1996: Manager of Blood Plasma Manufacturing Dept.
- 1997: Chief Scientist of Clinical development Dept.
- 1998: Manager of Blood Plasma Manufacturing Dept.
- 2004: Deputy General Manager of Blood Plasma Manufacturing Dept.
- 2005: General Manager of Blood Plasma Manufacturing Dept.
- 2007: General Manager of Quality Assurance Dept.
- 2010: General Manager of Vaccine Manufacturing Dept.
- 2013: Managing Director, Board of Directors, Deputy Senior Manager, Vaccine Division
( Other Experience and Professional Memberships)
- 2000-2005: Japan Blood Products Association Technical Committee
- 2001-2006: Japan Health Sciences Foundation Regulatory Standard Committee
- 2010-2014: Japan Vaccine Industry Association Technical Committee
(Honors)
- 1992: President Award for the Development of FVIII Concentrate“ Confact F”
- 1995: President Award for the Final Product Improvement of IVIG “Venilon”
- 2002: President Award for the Process Development and Patent Registration of Protein C Concentrate “Anact C”
(Some of the business achievement in the former company)
- Registration and Development of FVIII Concentrate“Confact F”
- Registration and Development of FIX Concentrate“Novact M”
- Registration and Technology Importation of ATIII Concentrate“Anthrobin P ”
- Registration and Final Product Improvement of IVIG “Venilon”
- Development of Hemophilia Inhibiter Concentrate“Byclot”
- Registration and Development of “Freeze-dried, cell culture-derived Japanese encephalitis vaccine (inactivated): ENCEVAC” in Japan and South Korea.
- Registration and Development of DPT-IPV 4 types combination vaccine
- Development of Vaccines, Blood products and Monoclonal products in Japan.
(Selected Peer-reviewed Publications)
- K.Tomokiyo1, Y.Ogata, et al., Large-scale production and properties of plasma-derived activated Factor VII concentrate; Vox Sanguinis, 84, 54-64,2003 Y.Nakatomi, Y.Ogata, et al., Combining FVIIa and FX into a mixture which imparts a unique thrombin generation potential to hemophilic plasma: an assessment of FVIIa/FX mixture as alternative bypassing agent.;Thromb Res ; 125:457-63,2010 Other (3)
(Oral Presentations and Poster Presentations)
- K.Tomokiyo, Y.Ogata, et al., Large-scale production and properties of human plasma-derived factor VIIa: Its application for a new bypass agent (FVIIa/FX mixture);19th International Society on Thrombosis and Haemostasis , Birmingham,2003Y.Ogata, Introduction of the latest Approved Drug Product “Dry Cell Culture Japanese Encephalitis Vaccine”, for Activation of Vaccine Development in Japan; The 9th Meeting of Biologics Forum, Tokyo, 2012 Other (10)
Access

BioShoot (Thailand) Co.,Ltd.
191 Silom Complex Building, 20th Floor, Room D, D1, D2,
Silom Road, Kwaeng Silom, Khet Bangrak, Bangkok Thailand 10500
- + 66 (0) 2-119-4661
- + 66 (0) 2-119-4660
- admin1@bioshoot.co.th
